The Inspire Sleep Apnea Implant is a medical device that is implanted into the body for the treatment of snoring and obstructive sleep apnea (OSA) syndrome.
Obstructive Sleep Apnea Syndrome
Obstructive sleep apnea syndrome is a disease caused by relapsing episodes of sleep apnea. This is a condition where breathing stops for more than 10 seconds, during which oxygen saturation in the blood is reduced by more than 4%.
OSA syndrome occurs during sleep, when the muscles of upper airways relax, causing the obstruction of the respiratory tract. This leads to deterioration of blood oxygenation and provokes short unconscious wake-up episodes throughout the night. An unhealthy pattern of sleep places stress on the body, causing increased blood pressure and development of severe diseases. The diseases caused by OSA can be physical, such as hypertension, myocardium infarction and stroke. They could also be mental, including depression, chronic fatigue syndrome, loss of memory and concentration.
Before any treatment, a sleep study or a polysomnography is needed. The polysomnography calculates the apnea index, which is the frequency of apnea episodes during one hour of sleep and determines the severity of the disease. The apnea index is considered significant if there are more than 20 apnea episodes per hour, regardless of the presence or absence of clinical symptoms.
The gold standard of treatment for OSA syndrome is CPAP-therapy. However, CPAP therapy is not for everyone as many people find it uncomfortable to sleep with the mask on the face and the machine itself can be loud, especially as it commonly sits near the head. Only 25% of patients can use CPAP-therapy for a long period of time.
Therefore, recently Inspire Medical Systems Inc. has come up with a very effective solution with the Inspire Sleep Apnea Implant.
How The Inspire Sleep Apnea Implant Works
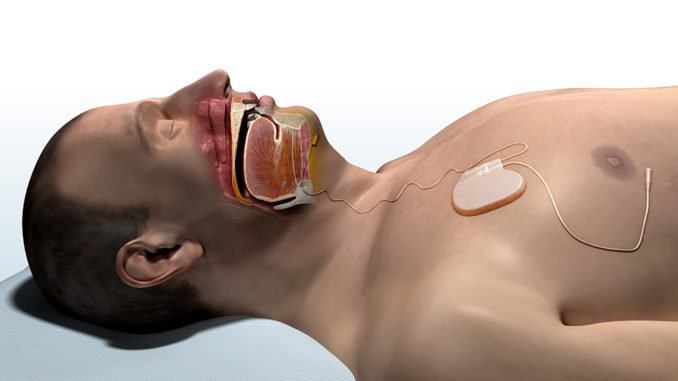
The Inspire Sleep Apnea Implant is a battery operated device that promotes electrostimulation of the sublingual nerve. The battery life is approximately 10 years.
The main unit of this device is placed under the skin, on the front right side of the chest and it monitors the phase of breathing. The other electrode is implanted for stimulation of the sublingual nerve, which creates muscle tension. This opens the upper airways and allows the tongue to stick out, and helps keep the airways open during sleep.
In addition to the two electrodes, this device also includes a handy small remote switch, which actually turns on the device before sleep and switches it off once awake. This disables the electrodes from disturbing the processes of communication and eating during the active phase of the day, when a person needs physiological narrowing of the larynx and tongue movements for these processes.
The patient may feel light involuntary contractions of the larynx, or tongue movements during initial use of the device. These movements do not cause pain, instead just a minor discomfort. These unpleasant effects disappear after the first few uses of the device.
Generally, the implant should be checked 1 or 2 times per year. However, depending on the situation, an Inspire therapy-trained doctor may consult patients more or less often.
The Inspire Sleep Apnea Implant is only suitable for:
- Patients who are more than 22 years old.
- Patients who have been diagnosed with moderate or severe obstructive sleep apnea syndrome.
- Patients with an apnea index in the range of 20 to 65.
- Patients who are not comfortable with using CPAP therapy or did not have the desired effect.
- Patients with a body mass index of less than 32.
Body mass index (BMI) = weight (kg) / height^2 (m2).
A person with a weight of 80 kg and a height of 170 cm has
BMI = 80kg / (1.7m ^ 2) = 80 / 2.89 = 27.68 kg/ m2
The Inspire Sleep Apnea Implant could potentially replace CPAP therapy as ‘the gold standard’ of treatment for obstructive sleep apnea syndrome. A study by Dr. Ryan J. et al. has shown that after implantation of the Inspire Sleep Apnea Implant, snoring is greatly decreased. A percentage of “no” or “soft” snoring changed from 22% at beginning of the treatment to 88% at 12 months and 91% at 24 months after the treatment. Also, the apnea index was reduced from 31 to 14. 82% of the patients in the study reported a nightly use of this therapy for five years. Therefore, it is evident that this method is highly effective and easy to use!
There was also another interesting study by Dr. Patrick J. from the Pittsburgh University Medical Center. The study found that stimulation of the sublingual nerve at the base of the tongue reduced the severity of obstructive sleep apnea by 68% and reduced the number of sleep apnea episodes from 29 to 9. Oxygen saturation of the blood also improved; daytime drowsiness decreased, and the quality of life of patients improved overall.
Inspire Sleep Apnea Cost
The Inspire Sleep Apnea Implant costs approx. $15,000-25,000, not including operational costs. Furthermore, the battery cannot be replaced after it is depleted and a new unit will need to be implanted. The implant can also move out of place over time. In comparison, the CPAP device costs approx. $1,500 to $ 3,000, which is much cheaper.
Inspire Sleep Apnea Side Effects
Implantation of the Inspire Sleep Apnea Implant is a surgical procedure with possible complications, as with any medical procedure. There are risks of infections after surgery, a temporary weakening of the tongue, post-operative bleeding, pain or discomfort in the implant area.
People with the following concerns are not suitable candidates for this device:
- Patients suffering from central apnea or mixed apnea with more than 25% of the total apnea index.
- Patients who have any anatomical issues that would compromise the performance of upper airway stimulation, such as the presence of a complete concentric collapse of soft palate.
- Patients with any existing condition or procedure that has compromised neurological control of the upper airway.
- Patients who are unable or do not have the ability to operate the sleep remote.
- Patients who are pregnant or planning to become pregnant.
- Patients with an implantable device that may be susceptible to unintended interaction with the Inspire system – allergic or rejection response to implanted materials.
The Inspire Sleep Apnea Implant therapy is FDA approved and proved to be an effective solution for obstructive sleep apnea syndrome. Currently, the therapy is being reviewed and approved by insurance companies on a patient-by-patient basis.
Speak to your doctor if this is a treatment you would like to explore further.
Proudly WWW.PONIREVO.COM